Abstract
BACKGROUND
Venetoclax (VEN), a once daily oral inhibitor of BCL2, achieves high response rates and acceptable toxicity in patients with relapsed or refractory (R/R) and treatment naïve (TN) CLL both as a single agent and in combination with anti-CD20 monoclonal antibodies such as obinutuzumab (OBIN), where minimal residual disease (MRD) negative responses have been observed in the majority of patients. Pre-clinical data suggest that concurrent treatment with the Bruton tyrosine kinase inhibitor ibrutinib (IBR) may further potentiate sensitivity to VEN. Given high individual response rates, largely non-overlapping toxicity, and distinct mechanisms of action, we initiated a phase 1b/2 study of combination OBIN, IBR, and VEN in CLL. A phase 1b study in R/R CLL patients established VEN 400mg daily as a safe dose for use in this combination. We report the initial results from the phase 2 cohort of TN patients.
METHODS
Patients with TN, symptomatic CLL meeting IWCLL 2008 guideline indications for therapy were eligible. Eligible patients had an ECOG PS ≤1 and preserved end-organ function, including creatinine clearance ≥50 mL/min/m2. Patients with chronic viral hepatitis infection, uncontrolled autoimmune cytopenia, and/or clinically apparent Richter's Transformation were excluded. Treatment was given at the doses and schedule established in the preceding phase 1b study (Jones ASH 2016). Treatment was planned for 14 cycles (C) of 28 days each with OBIN, IBR, and VEN started sequentially over the first 3 cycles. OBIN was started C1 (C1 D1: 100mg, C1 D2: 900mg, C1 D8,D15: 1,000mg, C2-8 D1: 1,000mg), IBR in C2 (C2-14 D1-28: 420mg), and VEN in C3 with dose escalation according to its US label (C3 20mg->50mg->100mg->200mg, C4-14 D1-28: 400mg). Risk for tumor lysis syndrome (TLS) with VEN was assessed according to US prescribing information. Adverse events (AEs) were assessed and graded using the NCI CTCAE v4.03 except hematologic AEs which were graded according to the IWCLL 2008 guidelines. Response was determined according to IWCLL 2008 criteria after cycles 8 and 14 with assessment for MRD in the bone marrow and peripheral blood by standard 4-color flow cytometry.
RESULTS
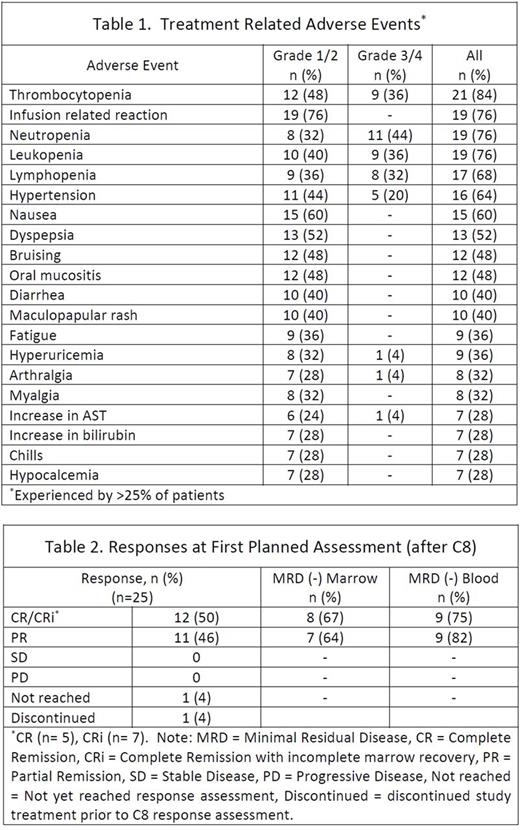
Enrollment was completed in November 2016 with 25 TN patients. The median age at enrollment was 59 (range 24-77) and 15 (60%) patients were men. Baseline risk characteristics included unmutated IGHV in 17 (71%) patients, complex karyotype in 6 (24%), FISH positive for del(17p) in 3 (12%), del(11q) in 5 (20%), del(13q) in 7 (28%), and tri(12) in 4 (16%). TLS risk was high for 7 (28%) and medium for 18 (72%) patients. In general, AEs were consistent with reports from the phase 1b study in R/R patients and the known safety profile of the individual agents. The most commonly experienced grade 1/2 treatment-related AEs were infusion reactions (76%), nausea (60%), and dyspepsia (52%). The most common grade 3/4 treatment-related AEs were neutropenia (44%), leukopenia (36%), thrombocytopenia (36%), lymphopenia (32%), and hypertension (20%). Hematologic toxicities were the most frequent high grade treatment-related AEs. There were no cases of neutropenic fever or TLS. Treatment-related AEs are in Table 1. To date 23 patients have reached the first (post-C8) response assessment with 1 patient leaving the study prior to C8 and 1 not yet reaching this time point. Of these 23 patients all had an objective response. The rate of complete remission (CR), including with incomplete marrow recovery (CRi), was 50% (12/24). MRD-negative status by flow cytometry in both blood and bone marrow was achieved in 14 (58%) patients; 3 CR, 5 CRi, 6 partial remission (PR). Responses at this early time point are summarized in Table 2. Twenty-two patients remain on study with 1 patient discontinuing treatment after C7 at the discretion of the treating physician, 1 patient after C10 electively, and 1 patient after C10 for the serious AEs of grade 3 colitis as well as grade 4 neutropenia which were both possibly related to study treatment.
CONCLUSIONS
OBIN, IBR, and VEN can be given safely without TLS and appears highly effective as initial therapy for CLL after response assessment at this initial (C8) time point. Objective responses, including MRD-negative complete responses, have been achieved in all patients to date and results for the primary endpoint of MRD (-) CR after C14 are expected in May 2018. Updated toxicity and response data will be presented for the 22 of 25 patients who remain on study.
Maddocks: Novartis: Research Funding; BMS: Research Funding; Pharmacylics: Research Funding; Merck: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal